
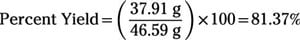
Percent yield chemistry calculator how to#
Related: Also find what is titration and how to calculate it using titration calculator.Thus, the percent yield in the given experiment is 102.9%. Since there's not a single chance of any human error while using this calculator, you will get the correct fractions for each entity.Researchers have to prepare a perfect product in a specific time this calculator saves your time from getting wasted in calculations. The time for in-lab synthetic work is limited.This calculator helps you to find the percent yield of a reaction using the percent yield equation.The yield percentage calculator makes you understand how you can figure out the percent yield of a reaction using the right formula.Don't forget, a minor error, even a single wrong decimal value, can alter the entire result.
Percent yield chemistry calculator manual#
If we are doing manual calculations, there is always a chance that we could make a mistake, the risk of making slight error is always present. Why should you use a percentage yield calculator? Related: Also use other online tools on this website like we offer molecular weight calculator and atomic mass calculator for free. Don't forget, getting yield more than or even equal to 100% is a red flag. After doing so, weigh the sample again, and you'll get the percent yield between 70%-90%. To settle this problem, filter your product to remove impurities or dry the end products to eliminate excessive solvent. It could happen when your sample or end product contains different solvents or impurities. Resultantly, the percent yield online calculator will display the accurate results.Īn important thing that needs to be noted here is getting the percent yield value of more than 100%.
Our yield percentage calculator utilizes the most credible formula to find out results. How does the percentage yield calculator work? Related: Find more useful calculators like we offer oxidation number calculator with solution and mass percent composition calculator which you can use for free. In the end, the same reaction is performed to give the desired yield of the selected products. If a response is not successful, the chemists change the reaction mechanism by changing its route. In simple words, chemists determine the percent yield of a reaction to predict the success rate of a chemical reaction. On the other hand, a reaction whose percent yield is more than eighty or ninety percent is much appreciated since it gives the maximum possible result. In such complex reaction, the cost of reactants and fuel would be much higher than the cost obtained by-products. The value of percent yield is quite essential since it tells whether a reaction would be successful or not.įor example, if the percent yield is less than fifty percent for a chemical reaction, it means that the response will not be feasible at all. Why is it important to calculate percent yield? There are useful blogs on this website which helps you learn how to balance complex chemical equations and you can also learn about balancing redox reactions in basic solutions from here.


 0 kommentar(er)
0 kommentar(er)
